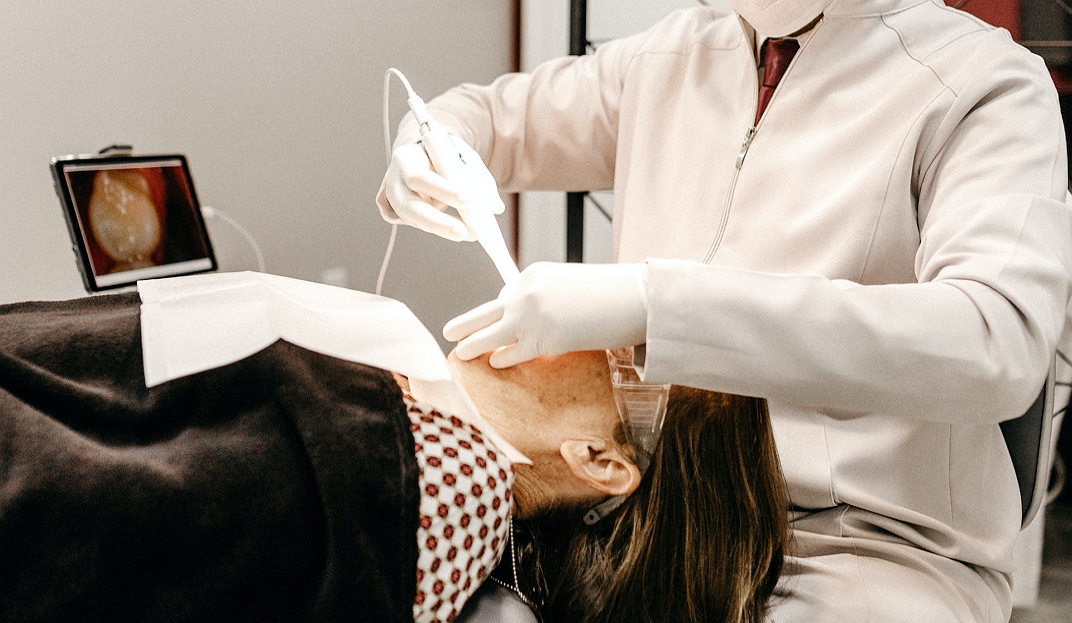
How to Stay Younger
Q: When senolytic drugs cause senescent cells to die, other (younger) cells need to divide and take the place of the dead cells. This cell division causes telomere shortening, thus possibly creating new senescent cells. How is it that the process of killing senescent cells is not self-defeating if new senescent cells are being created?
There are a couple of ways to come at this question. The first is to just look at the astonishing beneficial effects of senolytic drugs or gene therapies in aging mice and mouse models of age-related disease.1,2 In these studies, senolytic drugs have restored exercise capacity1 and capacity to form new blood and immune precursor cells3 in aging mice to near youthful norms, while preventing age-related lung hypofunction,4 fatty infiltration into the liver,5 weakening or failure of the heart,1,6,7 osteoporosis,8 and hair loss.9 These treatments have also prevented or treated mouse models of diseases of aging like osteoarthritis,10 fibrotic lung disease,11,12 nonalcoholic fatty liver disease (NAFLD),5atherosclerosis,13,14 cancer15 and the side-effects of conventional chemotherapy,2,16 as well as neurodegenerative diseases of aging like Parkinson’s17 and Alzheimer’s18,19,20 diseases… and on and on! So whatever collateral damage might ensue from ablating senescent cells, it’s pretty clear that senolytic treatments are doing a lot more good than harm.
But let’s drill down on the underlying reasoning of the question a little more. Suppose (as the question posits) that every time you destroy a senescent cell, a progenitor cell (one of the partly-specialized tissue-specific cells that repopulate a tissue with mature cells specific to that tissue) replicates to create a new cell to take its place. In fact, studies do show that when senescent cells are killed in a tissue, the progenitor cells begin to multiply and/or to function better as stem cells. This benefit is not due to the progenitor cells automatically replicating themselves and taking the place of the senescent cell, but because the baleful secretions spewed out of senescent cells inhibit the progenitor cells’ regenerative function, such that destroying senescent cells allows the progenitor cells to begin working properly again. This is observed in blood-cell-forming cells,3 cardiac progenitor cells,6 bone-forming cells,8and the cells that form new fat cells — in both mice21 and now (in a small, short-term clinical trial) even in humans!22
So does this support the worry behind the question? Not really. It just takes a moment’s thought to realize that just one such replication can’t possibly be enough to drive a stem/progenitor cell into senescence: if it did, of course, senolytic therapies would fail to reduce the net burden of senescent cells. But studies clearly show that administering senolytics does lower the overall number of senescent cells in aging and diseased tissues.
Also, if these drugs were not killing more senescent cells than they indirectly produced, you wouldn’t get relief from the harmful effects of having a high burden of senescent cells — and, of course, you do, in multiple tissues and in multiple models of aging and age-related disease.
Going Back to the Well for More
Still, even if a single round of senolytics isn’t enough to drive your stem cells senescent, what if you turn one tissue stem cell senescent for every two times they are triggered to proliferate by senolytic therapy — or every three, or four, or ten? Might a single round of senolytic drugs be a net benefit, whereas repeated treatments over a lifetime would deplete tissue stem cells step by step, eventually riddling the body with senescent cells and leaving the patient (murine or human) worse off over the long term?
Fortunately, we have long-term studies to address that question — and they tell us again that the answer is “no.”
In a study that played a critical role in launching the senolytic drug revolution,15 mice were engineered with a genetic self-destruct mechanism built into all of their cells, which would lie dormant until activated by a two-part command: one, the expression of the gene p16, which is characteristic of senescent cells; and two, an activating drug that scientists could administer to control the pace of senolytic activity. The researchers then waited until the animals were 12 months old (in human terms, this is similar to a person in his or her early 40s) before administering the drug for the first time. They then continued administering the drug every two weeks for the next six months — at which point the animals had received thirteen rounds of senescent cell-clearing therapy, and were roughly similar to humans in their mid-fifties.15
The study clearly showed that the animals benefitted from senolytic therapy, even after undergoing round after round of treatment across the span of their natural middle age through to early natural seniority.15 For instance, the animals whose senescent cell autodestruct mechanism had been triggered subsequently retained more functioning filtering units in their kidneys with age, and fewer of them died in middle age and early seniority.15
More directly on point with our question, the researchers looked to see the effects of multiple rounds of senolytic therapy on total senescent cell burden, and whether they substantially depleted the animals’ reserves of functionalprogenitor cells by forcing them to divide their way into senescent doom.14 To do this, the investigators looked at the effects of treatment on the preadipocytes (the progenitor cells that form fat cells (adipocytes)) in the animals’ fat tissue — a convenient place to look, because it’s easy to get at, and because it accumulates substantial numbers of senescent cells with age.
After some 13 rounds of senolytic therapy, the treated animals had only one eighth the number of senescentpreadipocytes as controls (Figure 1(a)) — a very substantial net reduction. Yet even so, the treated animals still had just as many functional progenitors left — or so close to as many that the difference was indistinguishable from chance (Figure 1(b)).15

Figure 1.
Activating senolytic “self-destruct” genes throughout midlife sustainably slashes senescent cell count (a) with no or very small effects on progenitor numbers (b) in adipose tissue. Bars in (b) with # are differences that were statistically significant. Redrawn from (15).In another study,23 researchers administered the natural senolytic compound fisetin to mice every single day, starting from the point when they were already in early seniority (and thus already had both a large number of senescent cells, and a dwindling supply of progenitor cells) and continuing on until their death. The extended treatment slashed the number of senescent cells in most tissues in the order of 50%. On top of that, the animals lived substantially longer, and their tissues suffered less severe age-related degenerative lesions than control animals.23
Out with the Old – and In With the New
That said, it is important to have plenty of functional cells around in order to reap the full benefits of senolytic therapy. This was illustrated in a study using senolytic drugs or “self-destruct” genes to treat animal models of osteoarthritis.10When joint disease was initiated by injury in young animals, the insult led to chronic joint damage and an accumulation of senescent cells in the synovium (the membrane surrounding the joint, where is the cells that maintain the complex fluid that lubricates the joint reside). Eliminating senescent cells reduced the inflammation in the joint, prevented joint erosion, and alleviated signs of pain in the animals.10
But senolytic treatment was much less effective when the scientists repeated the experiment in old animals, and the reasons why shed some light on our original question. Compared to young animals, the old animals accumulated many more senescent cells after their joints were injured, and those cells developed at deeper layers of the tissue, accompanied by more severe osteoarthritis.10 Perhaps this is because in the aging animals, more cells had already suffered significant aging damage, and were thus primed to be tipped over into senescence when injured. And when old animals were then administered senolytic treatment, the remaining healthy cells didn’t respond as well: the burden of zombie cells went down, and the old animals still got some pain relief, but genes that helped the young animals regenerate their damaged joints were not activated, and the cartilage quality score did not improve. The researchers suggest that this could be due to a decline in the number or functional capacity of the non-senescent cartilage-forming cells, driven by degenerative aging processes.10
Similarly, destroying senescent cells in aging mice reduced the excessive numbers of osteoclasts (cells that break down bone) that accumulate in aging bone, but did not restore the dwindling supply of bone-forming osteoblasts, lack of which no doubt constrained the rejuvenating effects of senolytic treatment in restoring the aging bone.8
In both of these cases, the lack of a boost to the number or activity of youthful cells was not the result of damage from the senolytic drug: the failing supply of such cells had already occurred before treatment was initiated. So the problem is not that senolytic therapies stop working or become counterproductive over time: rather, it’s that they only target one kind of aging damage, whereas aging drives disease and disability because of the accumulation of multiple kinds of cellular and molecular damage in our tissues with age. The solution is to pair the killing of senescent cells with the introduction of fresh, new functional cells via cell therapy. (See our analysis of a previous study on senolytic therapy in models of Parkinson’s disease for the clinical path ahead on such combination therapies).
And to return more directly to the original question, this would also be the solution if it ultimately turns out that many decades of of senolytic therapy really did drive too many tissue stem cells into senescence. But as we’ve seen, all the evidence suggests that this won’t be a problem during the decades of our current lifespans.